Invasion and Metastasis of Malignant Neoplasms
03/07/2025 Views : 70
Palagan Senopati Sewoyo
Neoplasm refers to an abnormal and excessive growth of new cells. This growth is uncontrolled and uncoordinated with the surrounding normal tissues. The newly formed tissue is disorganized and non-functional. These cells continue to replicate, completely ignoring the regulatory mechanisms that control normal cell growth. In general, neoplasm is also referred to as a tumor. A tumor is a swelling or mass, but not all swellings are tumors. In oncology, the term "tumor" specifically refers to a neoplastic mass that causes a visible or palpable lump on the body's surface. Neoplastic cells are those that have undergone transformation, usually due to genetic mutations, with DNA damage considered the primary cause.
Carcinogenesis, also known as oncogenesis, is the multi-step process through which cancer develops. For a cell to start dividing uncontrollably, the genes that regulate cell growth must become dysregulated. Two main classes of genes are involved in carcinogenesis: proto-oncogenes and tumor suppressor genes. When proto-oncogenes are activated by mutations, they send positive proliferative signals to the tumor. On the other hand, tumor suppressor genes act to inhibit neoplastic growth, and must be inactivated or lost in tumor cells. Generally, multiple mutations in these genes are required for a normal cell to become cancerous. These mutations provide the signals that trigger uncontrolled division in tumor cells.
In oncology, neoplasms are classified as either benign or malignant.
A tumor is considered benign if it remains localized, does not spread, can be
removed surgically, and does not cause death—unless its location interferes
with vital bodily functions. Malignant tumors, however, invade and destroy
surrounding structures, can spread to distant sites (metastasize), often recur
after removal or radiation, and may lead to death. Malignant tumors are
commonly referred to as cancer. The key differences between benign and
malignant tumors include differentiation and anaplasia, growth rate, local
invasion, and metastasis.
Metastasis is defined as the spread
of cancer from one organ to another or to a different part of the same organ.
Cancer cells can spread through one or more of the following metastatic routes:
- 1. Lymphatic
spread:
via lymphatic vessels, primarily through lymph nodes.
- 2. Hematogenous
spread:
through blood vessels to distant organs.
- 3. Transcoelomic spread: via direct contact between serosal surfaces in the body cavity.
Lymphatic spread involves invasion of lymphatic vessels and
transport through the lymphatic system to regional lymph nodes and distant
organs. This is often combined with hematogenous spread. In hematogenous
spread, tumor cells invade nearby blood vessels, enter the circulation, and are
transported to distant sites. A few tumors, such as canine osteosarcoma,
metastasize almost exclusively through the bloodstream and rarely involve lymph
nodes. Cancers like those of the pancreas, ovaries, and mesothelioma commonly
spread via transcoelomic metastasis, sometimes along with the other two
routes. Transcoelomic spread involves the direct implantation of tumor cells
onto the serosal surfaces of adjacent organs.
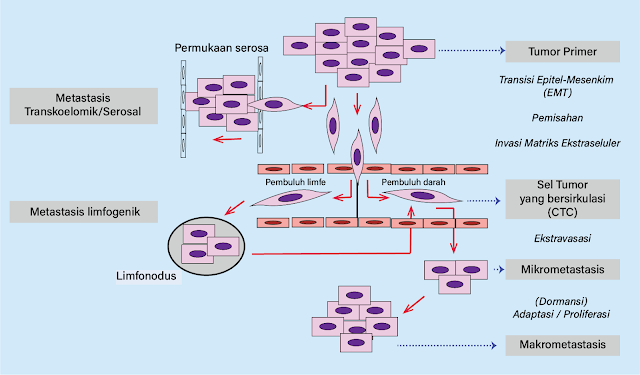
Figure 1. Metastasis cascade
Distant metastasis, primarily via hematogenous and often prior lymphatic spread, can be described as a metastatic cascade, consisting of five main steps:
- 1. Loss of adhesion between neighboring cells.
- 2. Invasion into the surrounding extracellular matrix and blood/lymphatic vessels—often involving epithelial-to-mesenchymal transition (EMT).
- 3. Survival in the bloodstream as circulating tumor cells (CTCs).
- 4. Extravasation and formation of micrometastases.
- 5. Development into macrometastases.
The first step in the cascade is the separation of potentially
metastatic cells from their neighbors, often associated with reduced expression
of cell adhesion proteins such as E-cadherin, leading to loss of
cell-cell contact.
Conversely, the expression of proteins involved in invasion and
migration through the extracellular matrix increases. These include CD44,
focal adhesion kinase (FAK), and matrix metalloproteinases (MMPs)
that help degrade the extracellular matrix. Detachment of neoplastic epithelial
cells and invasion into the surrounding matrix are associated with changes in
cell shape from a polygonal epithelial form to a spindle-shaped mesenchymal
form—this is known as epithelial-mesenchymal transition (EMT). It's
unclear whether mesenchymal tumors like sarcomas undergo EMT. This invasive
process is also influenced by surrounding stromal stem cells and macrophages,
which contribute to MMP production.
After invading the vessels, tumor cells are carried through the
bloodstream either individually or in small clusters, known as circulating
tumor cells (CTCs). Many efforts are underway to develop liquid biopsy
methods to detect CTCs in patient blood samples, which are less invasive and
more informative about disease status than traditional tumor biopsies. Early
studies show that CTCs from canine mammary tumors can be detected in
peripheral blood using markers such as CLDN7, CRYAB, ATP8B1, and EGFR.
Their presence is significantly and sensitively associated with metastatic
disease progression in dogs. Despite tumors shedding thousands to millions of
cells into circulation, the number of detectable CTCs is usually low—fewer than
10 CTCs per milliliter of blood and per million peripheral leukocytes.
Moreover, not all CTCs are clinically relevant. It’s estimated that fewer
than 0.1% of CTCs can actually lead to macrometastatic disease, and the
total number in the blood does not necessarily correlate with metastasis
development.
The question of why and where CTCs extravasate and form
metastases remains largely unanswered. However, it is clear that metastases
from different tumor types tend to follow specific patterns and affect certain
organs preferentially. For instance, canine mammary tumors and osteosarcomas
most commonly metastasize to the lungs, while feline lung carcinomas
often metastasize to the distal phalanges. In contrast, organs like the heart
or skin are rarely affected by metastasis. This pattern is explained by the
“seed and soil” theory: cancer cells (the seeds) can only grow into
metastases in suitable environments (the soil). A seed can only grow where the
soil supports it. The factors that make certain tissues or organs more
hospitable to metastasis are still under investigation. Identifying these
factors is of great interest because they could lead to the development of targeted
therapies to prevent metastatic disease. Among all organs, the bone
has been studied most extensively due to its relevance in human medicine.
Various mechanisms and factors are involved in tumor spread and bone
colonization, including secretion of chemokines (e.g., CXCL12, CXCL13)
and RANKL by osteoblasts and bone marrow stromal cells, which may
attract cancer cells to the bone marrow.
Micrometastasis formation is the next stage
following CTC extravasation. Micrometastases are more commonly observed
clinically than macrometastases. They consist of small clusters of tumor cells
that grow slowly or are dormant, and are often undetectable with standard
imaging techniques. In the next stage, micrometastatic tumors must adapt to
the new tissue microenvironment and grow into macrometastases—a process
called colonization. Colonization appears to be a major hurdle for tumor
cells. It’s believed that out of millions of tumor cells circulating in the
blood, only a few successfully form micrometastases. Most of them remain
dormant.
Dormancy is defined as a state in which cancer cells do not divide or
proliferate. During dormancy, cells stay in the G0 or G1 phase of the cell
cycle, waiting for signals to start proliferating again.
