Activated Carbon; The Black that Unique and Multi-purpose
28/06/2020 Views : 1275
Dewa Ngakan Ketut Putra Negara
Activated carbon (AC) or activated charcoal is a black solid a substance that can be in the form of powder, granular, or pellet and has an irregular crystalline structure (turbostratic structure) [1]. Activated carbon is a versatile adsorbent and is widely applied in various fields of life. The history of the use of activated carbon in medicine has been recorded since the time of Hippocrates which was used to relieve digestive problems and absorb the drug overdoses in the stomach [1]. Nowadays its use is increasingly widespread such as for water purification of pollutants [2], absorption of motor vehicle exhaust emissions [3], purification of biogas from CO2, as electrode and supercapacitor material [4], as nanocomposite material to absorb toxins such as mercury, eliminating sulfur from petroleum production [5] and for gas storage in ANG (adsorbed natural gas) technology [6].
Figure 1. (a) Powder AC (b) granular AC (c) turbostratic structure of AC [1]
Multi-function of activated carbon is due to its unique
characteristics; it has a high surface area of micropore (100 - 1000 m2
/ g), high micropore volume (0.15-5 cc / g) [1], and specific pore size
distribution so that it has a high adsorption capacity. The characteristics of
activated carbon are largely determined by the composition of the raw materials
(precursors) used and the parameters of the manufacturing process. Any material
containing carbon can be used as activated carbon. The biomass precursor is a
promising alternative precursor as a substitute for activated carbon sourced
from coal and petroleum residues that are decreasing. The precursors of the
biomass composition of lignin and cellulose have a very large influence on the
characteristics of activated carbon. Manufacturing of activated carbon consists
of the dehydration process (reduction of water content), carbonization (the
heating process to eliminate non-carbon elements using thermal decomposition
[7] and the activation process (the process of increasing the pore structure of
the charcoal resulting from the carbonization process). The activation process
can be carried out by physical method and chemical methods or a combination of
both methods. In chemical activation is involved chemical agents such as H3PO4,
NaOH,etc., as an activator.
As an example application, activated carbon from tabah
bamboo (Gigantochloa nigrociliata Buse-Kurz) is made by carbonizing at 800OC
for 2 hours, activating at 820OC for 50, 100, and 150 minutes and
flowing with nitrogen [8]. SEM image of activated carbon is shown in figure 2.
The surface characteristics produced are shown in table 1.
Table 1. The surface characteristic of activated carbon [8]


Figure 2. SEM image of activated carbons [8]
The effectiveness of adsorption is highly dependent on the characteristics of activated carbon and the type of substance to be absorbed (adsorbate). This due to each adsorbate (for example methane (CH4), mercury (Hg), sulfuric acid (H2S), carbon dioxide (CO2), etc.) has specific molecular dimensions. Only molecular sizes that are equal to or smaller than the pore diameter of activated carbon can be adsorbed by activated carbon, so to obtain optimal adsorption it is very important to know the characteristics of activated carbon and the type of substance to be absorbed. Activated carbon 100M-FA and 150M-FA have almost the same pore volume (Vp) and nitrogen absorption. Although 150M-FA has a higher pore start area (SBET) than 100M-FA, but because 100M-FA has a micro-sized pore diameter (Pz <2 nm) and is largely distributed by the micropore region (Figure 3), so that for methane storage application (CH4), 100M-FA activated carbon is more suitable. CH4 is more optimally absorbed in the pore size range from 0.5 nm to 1.1 nm [9]. 100M-FA activated carbon has a methane storage capacity of 0.167 kg per kg of activated carbon.
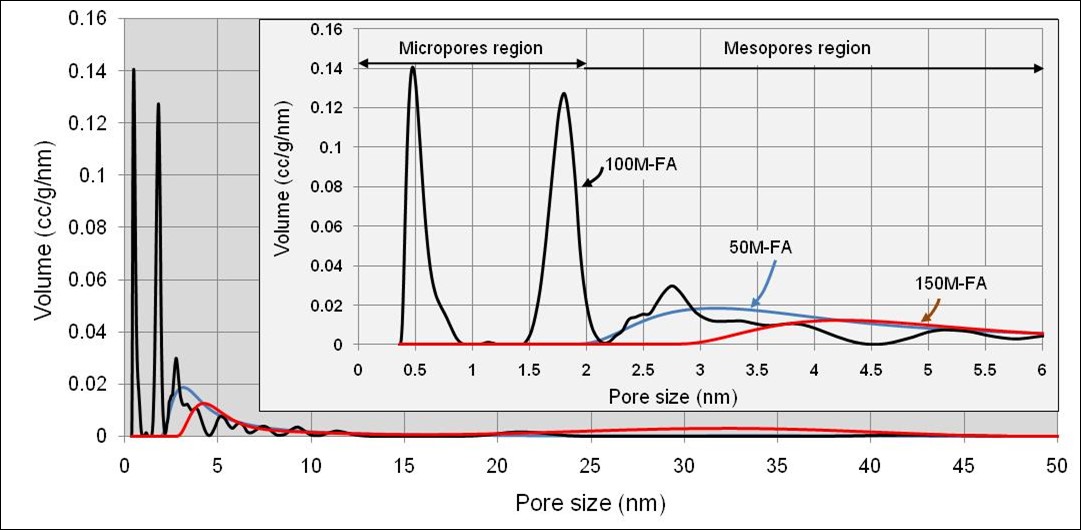
Figure 3. The pore size distribution of activated carbons
Activated carbon for methane storage application has promising prospects, especially for methane produced from biogas. So far, the methane produced from biogas in Bali can only be used at biogas locations. With the storage technology in this active carbon (ANG technology), biogas has the potential to be adsorbed, portable, and potentially commercially. However, further investigations are needed in efforts to find raw materials and optimal process parameters to obtain activated carbon with high storability.
References
[1] Bansal, Roop
Chand, and Meenakshi Goyal. 2005. Activated Carbon Adsorption. Taylor
& Francis Group, LLC. 6000 Broken Sound Parkway NW, Suite 300, Boca Raton,
FL 33487-2742, USA.
[2] T.A. Saleh, Isotherm, Kinetic, and
Thermodynamic Studies on Hg (II) Adsorption from Aqueous Solution by Silica-
Multiwall Carbon Nanotubes, a (2015), pp.16721–16731 https://doi.org/10.1007/s11356-015-4866-z.
[3] Viena V., Elvitriana, and Wardani S. (2018) ‘Application of banana peels waste as adsorbents for the removal of CO2,
NO, NOx, and SO2 gases from motorcycle emissions’, 3rd ICChESA 2017, IOP Conf. Series: Materials Science and
Engineering 334,
012037, pp. 1-8.
[4] W.
Cao, F. Yang, Supercapacitors from high fructose corn syrup-derived activated carbons,
Mater. Today Energy 9 (2018) 406–415 https://doi.org/10.1016/j.mtener.
2018.07.002.
[5] T.A. Saleh, Nanocomposite of carbon nanotubes
/ silica nanoparticles and their use for adsorption of Pb (II): from surface
properties to sorption mechanism, Desalin. Water Treat. (May) (2015) 37–41
https://doi.org/10.1080/19443994.2015.1036784.
[6] D.C.S. Azevedo, J. Cássia S. Araújo, M.
Bastos-Neto, A. Eurico B. Torres, E.F. Jaguaribe, C.L. Cavalcante, Microporous
Activated Carbon Prepared from Coconut Shells Using Chemical Activation with
Zinc Chloride, Microporous Mesoporous Mater. 100 (1–3) (2007) 361–364 https://doi.org/10.1016/j.micromeso.2006.11.024.
[7] Nor, N.M., Chung, L.L., Teong, L.K. and Mohamed, A.R. 2013. Synthesis of Activated
Carbon from Lignocellulosic Biomass and Its Applications in Air Pollution
Control : A Review. Journal of
Environmental Chemical Engineering. 1: 658–666.
[8] D.N.K. Putra Negara, T.G. Tirta Nindhia, I.W.
Surata, Fadjar Hidajat and Made Sucipta. Nanopore Structures, Surface
Morphology, and Adsorption Capacity of Tabah Bamboo-Activated Carbons. International Journal of Surfaces and
Interfaces 16 (2019) 22-28. https://doi.org/10.1016/j.surfin.2019.04.002
(Q2)
[9] Garcia Blanco, A.A., Alexandre de Oliveira,
J.C., Lopez, R. and Moreno-Pirajan,J.C. A Study of The Pore Size Distribution
for Activated Carbon Monoliths and Their Relationship with The Storage of Methane
and Hydrogen. Colloids and Surfaces A:
Physicochemical and Engineering Aspects. 357: 78-83.
